Phenoxyethanol(in 97,762 products) Banned by Gov or classified as a carcinogen by IARC
Banned by Gov or classified as a carcinogen by IARC
Banned by Gov or classified as a carcinogen by IARC
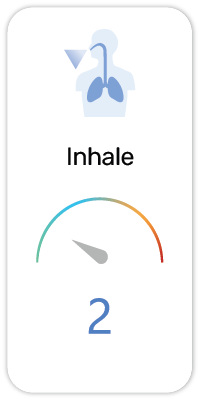
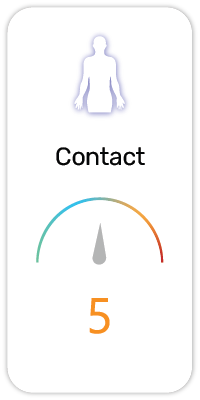
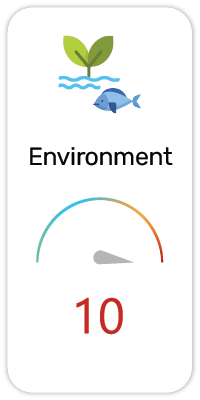
Potential Risk Index®:
About:
Functions:
1. Antimicrobial Preservative - Actively kills and inhibits the growth of unwanted microorganisms which may be harmful.
2. Antioxidant - Reduces oxidation to prevent the formation of free radicals which may be harmful to health.
3. Antiseptic / Disinfectant - Prevents growth of unwanted microorganisms.
4. Drug / Medicine - Treats, alleviates, cures, or prevents sickness. As officially declared by a governmental drug/medicine regulatory body
5. Fragrance / Fragrance Component - Provides or enhances a particular smell or odor.
6. Preservative - Prevents and inhibits the growth of unwanted microorganisms which may be harmful
Phenoxyethanol is a colorless liquid with a pleasant odor. It is a glycol ether used as a perfume fixative, insect repellent, antiseptic, solvent, preservative, and also as an anesthetic in fish aquaculture. [1]
Phenoxyethanol is an aromatic alcohol. It is both naturally found and manufactured synthetically. Demonstrating antimicrobial ability, phenoxyethanol acts as an effective preservative in pharmaceuticals, cosmetics, and lubricants. Phenoxyethanol is the most commonly used globally-approved preservative in personal care formulations. It is very easy to use in various types of formulations and is chemically stable. Phenoxyethanol is a colorless, clear, oily liquid with a faint aromatic odor at room temperature and a low water solubility and evaporation rate. It is produced by reacting phenol and ethylene oxide at a high temperature and pressure. This substance occurs naturally in green tea. [1]
Fun Facts:
-A good preservative against gram-negative and gram-positive bacteria (via gram staining, where a gram-negative bacteria will not be stained which indicates a greater likelihood of it being more resistant to antibiotics)
-Some blogs may claim that there is a link to ovarian cancer but the correlation has yet to be accepted by the scientific community as definitive
-An alternative to formaldehyde-releasing preservatives
Recent Findings:
Phenoxyethanol (2-Phenoxyethanol) products should not be applied to newborn infants of 26 weeks or younger, it was shown to penetrate the newborn’s skin and may cause central nervous system toxicity. [2] An 18-month old boy contracted generalized eczema during a routine administration of a DPT vaccine. Although the boy had a history of atopic eczema, the use of thiomersal as a preservative in a separate DPT vaccine resulted in no outbreaks. [3] This illustrates how some individuals may have an allergic reaction to phenoxyethanol despite its proven safety over thiomersal as a preservative.
Phenoxyethanol is rapidly absorbed through the skin within 24 hours. [4] Factoring in evaporative losses, approximately 88% of phenoxyethanol was absorbed after 48 hours. [5] Phenoxyethanol may cause a localized toxic sensation of burning and itching through the exposure of dermal tissues. [6] It can also be used to induce contact urticaria. [7] [8] Phenoxyethanol showed the lowest cytotoxicity out of most parabens and triclosan (propylparaben > butylparaben > ethylparaben > methylparaben > triclosan > phenoxyethanol), though human patch tests showed it to have a higher skin irritation potential compared to methylparaben and triclosan. [9]
In a published study in 2010, the study showed that out of all the ethylene glycol ethers, phenoxyethanol showed the most consistent cytotoxic effect on neurons and enhanced the hydrogen peroxide action. [10] This means that phenoxyethanol may be responsible for starting or make neuronal cell damage worse.
Phenoxyethanol has no statistically significant effects on the reproductive toxicity of mice. There are no differences between the control and sample group in sperm motility, sperm concentration and proportion of abnormal sperm. There were also no differences in white blood cell count, red blood cell count, packed cell volume or hemoglobin. [11] There is also no evidence of developmental toxicity observed based on a sample of New Zealand white rabbits. No external, visceral and skeletal abnormalities were observed. [11]
Low volatility of phenoxyethanol means that potential human exposure is most likely to occur via the dermal route. [12] White rabbits were dosed at 600mg/kg/day in order to observe the potential teratogenicity of phenoxyethanol. Darkened areas of skin were observed at the dermal application site, along with dark-colored urine, indicative of failing kidneys and livers being observed on days 11-18. [12] Red blood cell fragility is increased until no intact red blood cells can be detected which probably resulted in their deaths. Nevertheless, the fetuses from the dosed rabbits showed no abnormalities and phenoxyethanol did not affect the pregnancy rate nor body weight. [12]
Phenoxyethanol is often seen as a non-toxic alternative to formaldehyde. [13] [14] It is even used as an anesthetic in some species of animals like salmon. [15]
Overall, the EU SCCS has classified phenoxyethanol as safe for use as a preservative with a maximum concentration of 1.0%. However, care should still be taken when using products with phenoxyethanol, given its potential as a skin irritant.
Scientific References:
1. PubChem: https://pubchem.ncbi.nlm.nih.gov/compound/31236
2. Use of 2% 2-phenoxyethanol and 0.1% octenidine as antiseptic in premature newborn infants of 23–26 weeks gestation. (J. Hosp. Infect., 51(4), 305–307. DOI:10.1053/jhin.2002.1249)
3. Vogt, T., Landthalter, M., Stolz, W., 1998. Generalized eczema in an 18-month-old boy due to phenoxyethanol in DPT vaccine. (Contact Derm. 38 (1), 50–51)
4. Percutaneous penetration of 2-phenoxyethanol through rat and human skin. (Food Chem. Toxicol., 35(10-11), 1009–1016. DOI:10.1016/s0278-6915(97)00109-9)
5. Percutaneous penetration of 2-phenoxyethanol through rat and human skin. (Food Chem. Toxicol. 35 (10–11), 1009–1016.)
6. Inhibition of TRPV1 prevented skin irritancy induced by phenoxyethanol. A preliminary in vitro and in vivo study. (Int. J. Cosmet. Sci., 39(1), 11–16. DOI:10.1111/ics.12340)
7. Birnie, A.J., English, J.S., 2006. 2-Phenoxyethanol-induced contact urticaria. (Contact Derm. 54 (6), 349.)
8. Bohn, S., Bircher, A.J., 2001. Phenoxyethanol-induced urticaria. (Allergy 56 (9), 922–923.)
9. The Studies on the Development of Low Irritable Preservative System with Phenoxyethanol in Cosmetics (J. Soc. Cosmet. Chem. Korea 31(1,49), 43-49, http://www.koreascience.or.kr/article/JAKO200504840826183.page)
10. Effects of ethylene glycol ethers on cell viability in the human neuroblastoma SH-SY5Y cell line. (Pharmacol. Rep., 62(6), 1243–1249. DOI:10.1016/s1734-1140(10)70389-3)
11. Fragrance material review on 2-phenoxyethanol. (Food Chem. Toxicol., 50, S244–S255. DOI:10.1016/j.fct.2011.10.030)
12. Scortichini, B.H., Quast, J.F., Rao, K.S., 1987. Teratologic evaluation of 2-phenoxyethanol in New Zealand white rabbits following dermal exposure. (Fundam. Appl. Toxicol. 8 (2), 272–279)
13. Phenoxyethanol as a nontoxic substitute for formaldehyde in long-term preservation of human anatomical specimens for dissection and demonstration purposes. (Anat. Rec., 208(2), 271–278. DOI:10.1002/ar.1092080214)
14. Phenoxyethanol as a Nontoxic Preservative in the Dissection Laboratory. (Cells Tissues Organs, 136(2), 155–158. DOI:10.1159/000146816)
15. 2-Phenoxyethanol as a General Anaesthetic for Sockeye Salmon. (Journal of the Fisheries Research Board of Canada, 20(6), 1435–1440. DOI:10.1139/f63-097)
16. Phenoxyethanol: Protein Preservative for Taxonomists., (Science, 163(3868), 681–683. DOI:10.1126/science.163.3868.681)
17. Methyldibromoglutaronitrile (Euxyl K 400): An important “new” allergen in cosmetics. (J. Am. Acad. Dermatol., 35(5), 743–747. DOI:10.1016/s0190-9622(96)90730-6)
18. Ecotoxicity and screening level ecotoxicological risk assessment of five antimicrobial agents: triclosan, triclocarban, resorcinol, phenoxyethanol and p-thymol. (J. Appl. Toxicol., 1222–1229. DOI:10.1002/jat.2771)
19. Validation of HPLC Analysis of 2-Phenoxyethanol, 1-Phenoxypropan-2-ol, Methyl, Ethyl, Propyl, Butyl and Benzyl 4-Hydroxybenzoate (Parabens) in Cosmetic Products, with Emphasis on Decision Limit and Detection Capability (Chromatographia, 2004, 59, 47-53. DOI: 10.1365/s10337-003-0127-2)
Regulatory References:
1. South Korea - Ministry of Food and Drug Safety - Prohibited/Restricted Chemicals
- Ref: 1120
2. Palau Sunscreen Ban (Senate Bill No. 10-135, SD1, HD1), https://www.icriforum.org/the-republic-of-palau-bans-sunscreen-chemicals-to-protect-its-coral-reefs-and-unesco-world-heritage-site/ - Phenoxyethanol
3. Japan - Ministry of Health, Labour and Welfare - Restricted Ingredients
- Phenoxyethanol
4. EU Scientific Committee on Consumer Safety
5. EU CosIng Annex V, PRESERVATIVES ALLOWED IN COSMETIC PRODUCTS [2017]
- Ref: V/29
6. Association of Southeast Asian Nations Annex VI, Allowed Preservatives
- 2-Phenoxyethanol
7. International Fragrance Association Transparency List [2015]
- 2-Phenoxyethanol
8. European Chemicals Agency
Safety and Hazards (UN GHS):
1. Harmful if swallowed (H302)
2. Causes skin irritation (H315)
3. Causes serious eye irritation (H319)
4. May cause drowsiness or dizziness (H336)
Potential Health Concerns For:
1. Dermatitis, Atopic (PubMed ID:15218738)
User Comments:
Submit